Key points
- Private health insurers and an independent scientist are calling on Medicare to stop funding spinal cord stimulators.
- The expensive devices are designed to interfere with nerve signals to treat stubborn pain conditions.
- New evidence suggests they are no better than a placebo for a key pain condition.
Private health insurers are calling for Medicare to stop funding spinal cord stimulators after a controversial new study concluded the expensive devices were no better than a placebo for treating a key type of chronic pain.
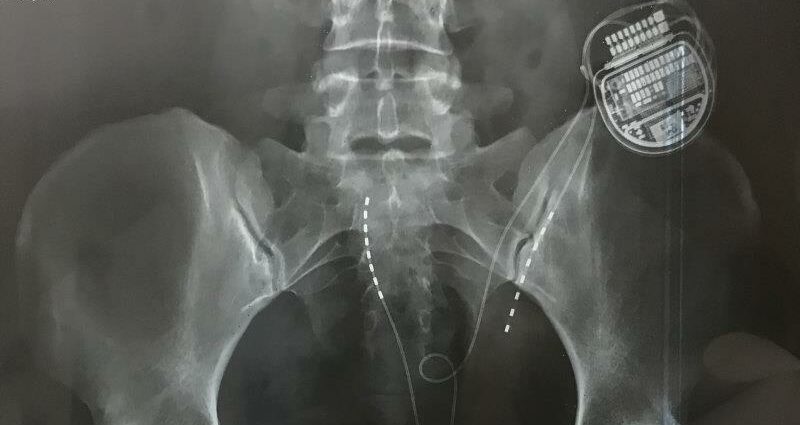
The stimulators are small computers surgically wired into the spinal cord. They are mostly used to treat chronic pain or back pain, in theory by sending electrical pulses to mask or interrupt nerve signals before they get to the brain.
An x-ray showing a spinal cord stimulator in situ.
Their use is growing fast, with the global industry expected to be worth almost $3 billion USD by 2025.
Studies have shown they are safe and effective. But independent scientists argue those studies are biased because they are funded by the manufacturers of the devices or run by surgeons who have received industry funding.
A paper published in leading journal JAMA last week found a stimulator was no better than a placebo for chronic back and leg pain following a spinal surgery.
“This is a strong signal this treatment may not work,” said the Institute for Musculoskeletal Health’s Dr Adrian Traeger, who has been writing a review of the evidence for spinal cord stimulators to treat back pain.
He called on Medicare to stop subsidising the surgery until more evidence was collected. “Really, if these are being provided, it should only be in the context of a clinical trial,” Traeger said.
But the Neuromodulation Society of Australia and New Zealand immediately hit out at the trial.
“This study … doesn’t remotely represent what we do for our patients and doesn’t reflect the clinical outcome that we see in our patients,” said president Dr James Yu.
The researchers studied 50 patients in Norway left with chronic pain after spinal surgery.
Adam Young holds his spinal cord stimulator in his hand. He says the device never gave him any relief.Credit:Paul Harris
Some modern spinal cord stimulators use “burst” stimulation, which means patients cannot directly tell if the stimulator is switched on or not.
In the recent study, each patient had a stimulator implanted, then spent six months with it turned on, and – without the patients knowing – another six months with it essentially turned off.
It made no difference. The researchers found there were no significant differences in scores for pain, disability or quality of life if the stimulator was on or off.
Warburton-based photographer Lou Whelan has twice had a spinal cord stimulator implanted to treat pain from a failed spine surgery.
“Both were abject failures,” she said. “The device has never been effective. It’s sitting in my spine, switched off and causing worsening epidural scarring.”
An investigation by The Age in February revealed spinal cord stimulators, or the operations used to install them, had been linked to more than 500 serious complications reported to regulators – 79 per cent severe and 13 per cent life-threatening.
At the time, manufacturers and representatives of the surgeons who install them said the devices were safe and effective, pointing out data did not show the complications were directly caused by the device.
“Nowadays with the new technology, we can expect up to 80 per cent pain control or more,” Dr Nick Christelis, then-president of the Neuromodulation Society of Australia, told The Age at the time. “The complication rate is about one in 10 over the lifespan of the device.”
The Age’s investigation prompted Private Healthcare Australia, the peak body for Australia’s private health insurers, to look into their own members’ outcomes.
“At this stage, it looks as though for the great majority of patients, this technology is little more than a very expensive placebo,” said CEO Dr Rachel David. In one case, she said, the investigation revealed more than $400,000 had been spent on a patient who ended up “no better off”.
She called for Medicare to stop funding the procedure, a position Traeger agrees with.
Three powerful government bodies are also currently reviewing spinal cord stimulators: the Therapeutic Goods Administration; the Australian Commission on Safety and Quality in Healthcare; and, the Prosthesis List Advisory Committee.
“The use of stimulators should only be considered following full consultation with the treating physician and informed consent, taking into consideration all associated risks and all other potential treatment options,” a spokeswoman for the health department said.
The Morning Edition newsletter is our guide to the day’s most important and interesting stories, analysis and insights. Sign up here.
Most Viewed in National
From our partners
Source: Read Full Article
-
The shopping trolley essentials that have DOUBLED in one year
-
Pros and cons of Polaris Bios Editor
-
Charlotte Sena is found ALIVE two days after nine year-old vanished
-
Revelation Nicola Bulley struggled with alcohol cleared with family
-
Polish woman claiming to be Madeleine McCann gets answers as DNA results in