Fennec Pharmaceuticals Inc. (FENC), a specialty pharmaceutical company, anticipates FDA decision on its investigational drug PEDMARK, proposed for the prevention of platinum-induced ototoxicity in pediatric patients one month to less than 18 years of age with localized, non-metastatic, solid tumors.
The decision date is due on September 23, 2022.
PEDMARK is a unique formulation of Sodium Thiosulfate (STS).
The FDA had twice issued Complete Response Letter (CRL) for PEDMARK for the prevention of platinum-induced ototoxicity in pediatric patients – on August 11, 2020, then again on November 30, 2021.
The Resubmitted New Drug Application was based on two Phase 3 clinical studies of survival and reduction of ototoxicity, the Clinical Oncology Group (COG) Protocol ACCL0431 and SIOPEL 6.
The COG ACCL0431 protocol enrolled childhood cancers typically treated with intensive Cisplatin therapy for localized and disseminated disease. SIOPEL 6 enrolled only hepatoblastoma patients with localized tumors.
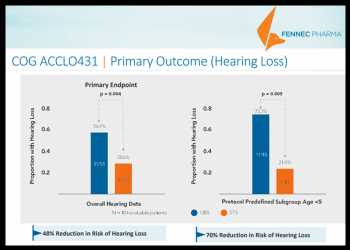
In the COG ACCLO431 trial, which involved 104 evaluable patients, 56% in the control arm experienced hearing loss while only 28.6% in the treatment arm of Pedmark experienced hearing loss. The results imply a 48% reduction in the risk of hearing loss with Pedmark, according to the company.
Similar to the COG trial, there was a 48% decrease in the risk of hearing loss in the SIOPEL 6 study as well.
The company estimates that over 10,000 children receive platinum-based chemotherapy annually in the U.S. and Europe. The incidence of ototoxicity depends upon the dose and duration of chemotherapy, and many of these children require lifelong hearing aids.
There is no FDA-approved preventive agent for platinum-induced ototoxicity and only suboptimal cochlear (inner ear) implants have been shown to provide some benefit.
FENC has traded in a range of $3.82 to $10.08 in the last 52 weeks. The stock closed Friday’s trading at $6.61, down 1.64%.
Source: Read Full Article
-
Will Financial Stress Break The Global Economy?
-
North American Leaders' Summit Pledge Cooperation On Semiconductors
-
JioMart morphs into e-marketplace to take on Amazon and Flipkart
-
Disney Skewers Nelson Peltz As Lacking “Skills And Experience To Assist Board”; Reveals Marvel Chair Isaac Perlmuttter Backed Activist Investor
-
Asian Markets Trading Mixed