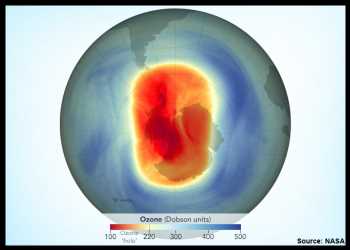
The Antarctic ozone hole reached its maximum size of the year on September 21, according to annual satellite and balloon-based measurements made by NASA and NOAA.
At 10 million square miles, or 26 million square kilometers, the hole ranked as the 12th largest single-day ozone hole since 1979.
During the peak of the ozone depletion season from September 7 to October 13, the hole this year averaged 8.9 million square miles, which is approximately the size of North America.
“It’s a very modest ozone hole,” said Paul Newman, leader of NASA’s ozone research team and chief scientist for Earth sciences at NASA’s Goddard Space Flight Center in Greenbelt, Maryland. “Declining levels of human-produced chlorine compounds, along with help from active Antarctic stratospheric weather slightly improved ozone levels this year.”
The ozone layer acts like Earth’s natural sunscreen, as this portion of the stratosphere shields the planet from the Sun’s harmful ultraviolet radiation. A thinning ozone layer means less protection from UV rays, which can cause sunburns, cataracts, and skin cancer in humans.
Every September, the ozone layer thins to form an “ozone hole” above the Antarctic continent. The hole isn’t a complete void of ozone; scientists use the term “ozone hole” as a metaphor for the area in which ozone concentrations above Antarctica drop well below the historical threshold of 220 Dobson Units.
Antarctic ozone depletion occurs when human-made chemicals containing chlorine and bromine first rise into the stratosphere. These chemicals are broken down and release their chlorine and bromine to initiate chemical reactions that destroy ozone molecules. The ozone-depleting chemicals, including chlorofluorocarbons (CFCs), were once widely used in aerosol sprays, foams, air conditioners, fire suppressants, and refrigerators.
The 1987 Montreal Protocol and subsequent amendments banned the production of CFCs and other ozone-destroying chemicals worldwide by 2010. The resulting reduction of emissions has led to a decline in ozone-destroying chemicals in the atmosphere and signs of stratospheric ozone recovery.
Source: Read Full Article
-
David Neumann’s Newmation Signs Malenga Mulendema, Creator Of Netflix’s First African Animated Series ‘Supa Team 4’
-
European Shares Seen Opening Higher After Failed Wagner Mutiny
-
UK Halifax House Prices Rise For Second Month
-
Review: The electric Toyota BZ4X is perfectly fine
-
Elton John Says ‘The Devil Wears Prada’ Musical “Not Ready” For Post-Chicago Stagings